Learning Objectives
- Define the atomic number.
- Relate the number of electrons in an element to the atomic number for that element.
Medical Definition of atomic number: an experimentally determined number characteristic of a chemical element that represents the number of protons in the nucleus which in a neutral atom equals the number of electrons outside the nucleus and that determines the place of the element in the periodic table — see Chemical Elements Table. The atomic number (represented by the letter Z) of an element is the number of protons in the nucleus of each atom of that element.An atom can be classified as a particular element based solely on its atomic number. The atomic number is simply the number of protons in an atom. For this reason, it's sometimes called the proton number. In calculations, it is denoted by the capital letter Z. The symbol Z comes from the German word zahl, which means number of numeral, or atomzahl, a more modern word which means atomic number. The atomic number of an element is equal to the total number of protons in the nucleus of the atoms of that element. The atomic number can provide insight into the electronic configuration of the element. For example, carbon has an electron configuration of He 2s 2 2p 2, since its atomic number is 6. What is the atomic number and mass number?
The atomic number is the number of protons an atom has. It is characteristic and unique for each element. The atomic mass (also referred to as the atomic weight) is the number of protons and neutrons in an atom. Atoms of an element that have differing numbers of neutrons (but a constant atomic number) are termed isotopes. Isotopes, shown in.
What is unique about each one of us?
For the vast majority of people, it is not their name, because it is quite possible for others in the world to have the same name (check it out by doing an internet search for your name and see how many other of “you” there are). It is not your physical description. Eye-witnesses to crime scenes often pick the wrong person when trying to identify the criminal.
There may be some unique identifiers for us. If you have a cell phone in your name, nobody else in the world has that number. Email addresses are different for each of us, which is a good thing since we can email almost anywhere in the world. Our DNA is unique, but getting a DNA analysis is expensive and time-consuming, so we really don’t want to have to explore that.
Organizing the Elements
One of the goals of science is to discover the order in the universe and to organize information that reflects that order. As information about the different elements was made known, efforts were made to see if there were patterns in all of the data. An early attempt to organize data was made by Mendeleev, who developed the first periodic table. His data set was based on atomic weights and was instrumental in providing clues as to the possible identity of new elements. Once we learned the details of the atomic nucleus, the table was based on the number of protons in the nucleus, called the atomic number of the element.
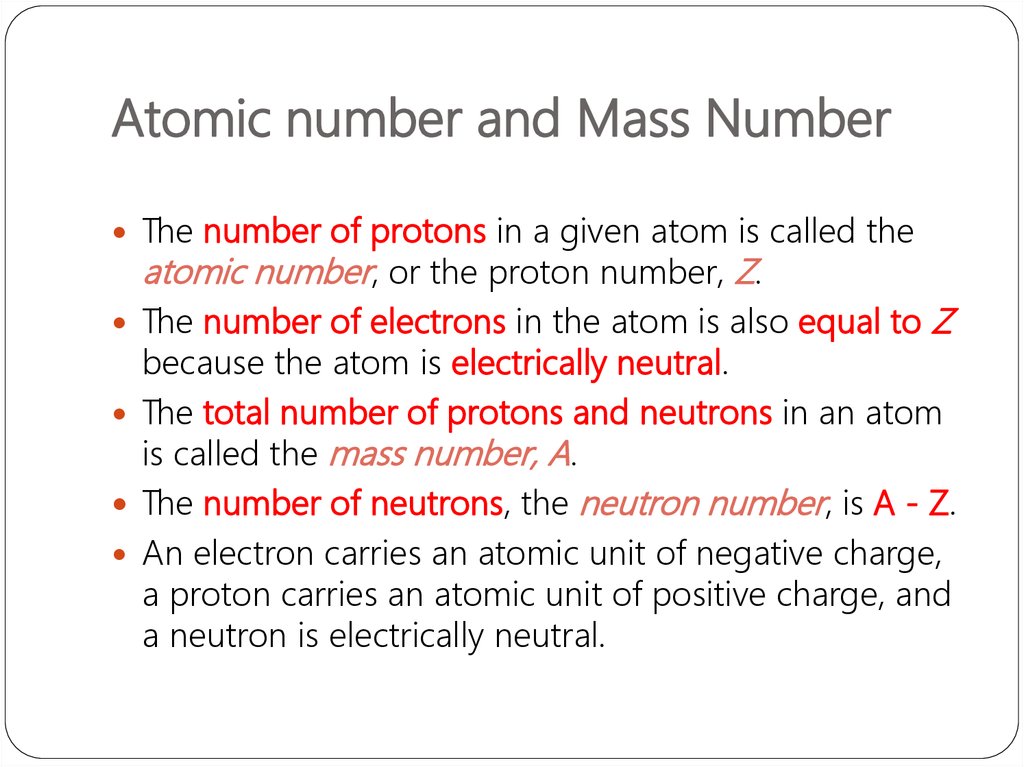
Atomic Number
Figure 1. How can you determine the atomic number of an element?
The atomic number (Z) of an element is the number of protons in the nucleus of each atom of that element. This means that the number of protons is the characteristic which makes each element unique compared to all other elements. Elements are different because of their atomic number. The periodic table displays all of the known elements and is arranged in order of increasing atomic number. In this table, an element’s atomic number is indicated above the elemental symbol. Hydrogen, at the upper left of the table, has an atomic number of 1. Every hydrogen atom has one proton in its nucleus. Following on the table is helium, whose atoms have two protons in the nucleus. Lithium atoms have three protons, and so forth.
Atomic Number Is The Number Of What In An Atom
Since atoms are neutral, the number of electrons is equal to the number of protons. Hydrogen atoms all have one electron occupying the space outside of the nucleus. Manganese (atomic number 25) would have twenty-five protons and twenty-five electrons.
Figure 2. The periodic table classifies elements by atomic number.
The classification of elements by atomic number allows us to understand many properties of the atom and makes it possible to predict behaviors instead of just having to memorize everything.
Summary
- The atomic number (Z) of an element is the number of protons in the nucleus of each atom of that element
- The number of electrons is equal to the number of protons in an element.
Practice
Atomic Number And Mass Number Worksheet
Use the link below to answer the following questions:
- What letter is used by convention to designate the atomic number?
- What determines the chemical properties of an element?
- What are the atomic numbers of the elements that appear in nature?
- How many elements were known in John Dalton’s day?

Atomic Number Is The Number Of Proton
Review
Atomic Number Is The Number Of Electrons
- Name two unique identifiers of people.
- Who developed the first periodic table?
- What was this table based upon?
- What is the current periodic table based upon?
- What does the atomic number represent?
- How many protons are in the following elements:?
- Ne
- Ca
- Pt
- Write the symbol for the element with the following atomic number:
- 18
- 41
- 82
- 12
Glossary
- periodic table: This table displays all of the known elements and is arranged in order of increasing atomic number.
- atomic weight: Each chemical element has an atom with a given mass.
- atomic number : The number of protons in the nucleus of each atom of that element.
Atomic Number Chart
References
How To Find Atomic Number
- Laura Guerin, based on image by User:Materialscientist/Wikimedia Commons. CK-12 Foundation (original image: http://commons.wikimedia.org/wiki/File:Atomic_number_depiction.jpg).
- User:Cepheus/Wikimedia Commons. http://commons.wikimedia.org/wiki/File:Periodic_table.svg.
